Title
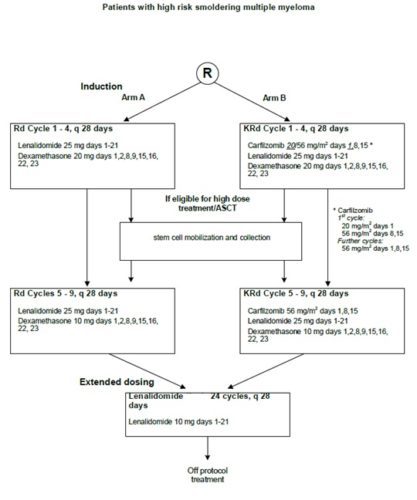
Carfilzomib, Lenalidomide and Dexamethasone versus Lenalidomide and Dexamethasone in High- Risk Smoldering Multiple Myeloma: A Randomized Phase 2 StudyStudy map
Overview / Summary
Study details
Patient eligibility criteria
- Patients must have histologically or cytologically confirmed Smoldering Multiple Myeloma based on the 2014 International Myeloma Working Group Criteria:
- Serum M-protein ≥30 g/L, or urinary monoclonal protein >500 mg per 24 hours, and/or monoclonal bone marrow plasma cells ≥10-60 %
- Absence of CRAB symptoms:
– anemia: Hemoglobin <6.2 mmol/L (10 g/dl) or a hemoglobin value of >1.2 mmol/L (2 g/dL) below the lower limit of normal
– renal failure: serum creatinine > 2.0 mg/dL or creatinine clearance < 40 ml/min
– hypercalcemia: serum calcium >0·25 mmol/L (>1 mg/dL) higher than the upper limit of normal or >2·75 mmol/L (>11 mg/dL)
– Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT
- Absence of myeloma defining events:
– Involved/uninvolved serum free light chain ratio ≥100 with involved free light-chain concentration ≥10 mg/dl
– Presence of 2 or more focal lesions by MRI (2 of which at least 5 mm)
– Clonal bone marrow plasma cell percentage ≥60%
- Patients must have high risk Smoldering Multiple Myeloma based on the Mayo Clinic and/or the PETHEMA criteria:
- 3 factors of Mayo Clinic criteria:
– Bone marrow plasma cells ≥10 %
– Serum M-protein ≥ 3 g/dl
– Serum free light-chain ratio <0.125 or >8
- And/Or 2 factors of PETHEMA criteria:
– Of the plasma cell population ≥95% abnormal plasma cells (presence or absence of CD38, CD56, CD19 and/or CD45)
– Immunoparesis, a reduction (below the lower normal limit) in the levels of 1 or 2 of the uninvolved immunoglobulins (Ig) (an exception can be made in FLC disease in which all three uninvolved immunoglobulins can be below the lower limit of normal)
- Measurable disease defined by any one of the following:
- Serum monoclonal protein ≥ 1.0 g/dl
- Urine monoclonal protein >200 mg/24 hour
- Serum immunoglobulin free light chain >10 mg/dL AND abnormal kappa/lambda ratio (reference 0.26-1.65)