Title
A multicenter, open label, randomized phase ii study comparing daratumumab combined with bortezomib-cyclophosphamide-dexamethasone (dara-vcd) versus the association of bortezomib-thalidomide-dexamethasone (vtd) as pre transplant induction and post transplant consolidation, both followed by a maintenance phase with ixazomib alone or in combination with daratumumab, in newly diagnosed multiple myeloma (mm) young patients eligible for autologous stem cell transplantationStudy map
Overview / Summary
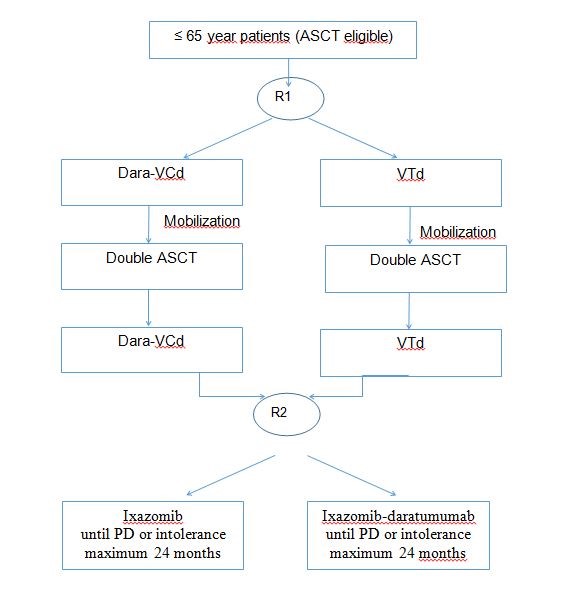
Primary obiectives of part 1 of the study (e.g. induction+ASCT+consolidation) will be the following:
- To compare the efficacy of Dara-VCd to that of VTd in terms of progression free survival (PFS) at 3 years from first randomization (24 months from second randomization)
- To compare the efficacy of Dara-VCd to that of VTd in terms of minimal residual disease (MRD) negativity rate (≥10-5 sensitivity level) after induction and consolidation therapy
Primary objectives of part 2 of the study (e.g. maintenance) will be the following:
- To compare the efficacy of daratumumab-ixazomib to that of single-agent ixazomib in terms of MRD negativity rate (≥10-5 sensitivity level) and probability of conversion from MRD positivity (10-3 and 10-4 sensitivity levels) to MRD negativity (≥10-5 sensitivity level) by the end of maintenance therapy
- To compare the efficacy of daratumumab-ixazomib to that of single-agent ixazomib in terms of PFS at 2 years from second randomization to maintenance therapy.
Study details
Participating countries:
- Czech Republic (6 sites)
- Italy (19 sites)
- Greece (3 sites)
Patient eligibility criteria
About 400 newly diagnosed young multiple myeloma patients.
- Patient at least 18 years of age and ≤ 65 years.
- Patient eligible for ASCT.
Publications
No publications connected to this trial at the moment