Title
Phase 2 Study Applying Innovative Minimal Residual Disease (MRD) Techniques for Participants with Previously Untreated Multiple Myeloma Treated with Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone (D-VRd) Prior To and After High-dose Therapy Followed by Autologous Stem Cell Transplantation (ASCT) - TAURUSStudy map
Overview / Summary
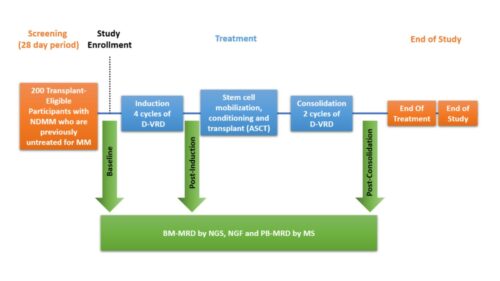
200 subjects will be enrolled in a single arm treatment with Daratumumab, Velcade (Bortezomib), Lenalidomide and Dexamethasone. D-VRd will be administered for 4 induction cycles prior to and 2 consolidation cycles following autologous stem cell transplantation. Samples will be obtained post-
induction and post-consolidation to evaluate Mass Spectrometry Minimal Residual Disease (MS-MRD) using blood samples to compare it with the Minimal Residual Disease (BM-MRD) using bone marrow samples. There will be no maintenance or FU in this study. The primary endpoint is to compare MRD measurements by NGS (BM) and MS (M-protein measurement in peripheral blood [PB]) at post-consolidation timepoint in transplant-eligible NDMM participants treated with D-VRd prior to and after ASCT.
Study details
Patient eligibility criteria
Patient eligibility criteria:
- 18 to 70 years of age, inclusive.
- Must have a new diagnosis of MM as per IMWG criteria.
- Measurable disease
- Newly diagnosed and treatment-naïve participants for whom high-dose therapy and autologous stem cell transplantation is part of the intended treatment plan.
- Eastern Cooperative Oncology Group (ECOG) performance status score of 0, 1, or 2.
- Clinical laboratory values meeting the required criteria during screening and ≤3 days prior to receiving first study treatment dose.
- Adequate bone marrow function.
- Adequate liver function.
- Adequate renal function.
- A female of childbearing potential (FOCBP) must have two negative serum or urine pregnancy tests at screening including within 24 hours of the start of study treatment.
- Willing to practicing at least 1 highly effective method of contraception starting 4 weeks prior to start of study treatment, while receiving study treatment including during any dose interruptions, and for at least 3 months after the last dose of any component of the study treatment.