Title
EMN Biobanking ProjectStudy map
Overview / Summary
This is an observational, non-interventional, multicenter study for the prospective collection, storage and analysis of patients’ biological samples.
Patients will be enrolled in the biobanking project after the signature of the specific Informed Consent Form (ICF) and the confirmation of the eligibility criteria. Then, they will be treated according to Standard of Care (SOC) or trial specific therapy.
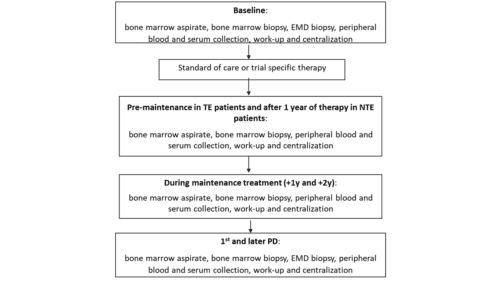
During the study, samples will be real-time locally collected according to the table “Study assessments” below, and then batch-wise shipped and centrally stored in the EMN central laboratories.
Study objectives
This study establishes a common international infrastructure useful to collect standard clinical variables at baseline and during treatment and to uniformly collect and store biological samples from patients at baseline, after ASCT/End of Induction (NTEMM), at the time of 1st and 2nd PD.
This study aims at:
- allowing analyses for better prognostic models;
- allowing MRD evaluation by NGS in the future, inside and outside clinical trials;
- improving understanding of disease initiation and progression;
- improving understanding of development of resistance to specific agents.
Study population: 6000 patients.
Expected project duration: 5 years
Participating countries:
Italy (active)
Czech Republic (noy active yet)
Greece (not active yet)
Austria (not active yet)
Serbia (not active yet)
Study details
Patient eligibility criteria
- Subjects with MGUS, SMM, MM, PCL, EMD;
- Subjects are ≥ 18 years old;
- Subjects have provided written informed consent in accordance with federal, local, and institutional guidelines prior to initiation of any study-specific activities or procedures.
- Subjects do not have kind of condition that, in the opinion of the Investigators, may compromise the ability of the subjects to give written informed consent and subjects are, in the investigator(s) opinion, willing and able to comply with the protocol requirements