Title
Phase 2 study of daratumumab monotherapy in previously untreated patients with stage 3B light chain (AL) amyloidosis The Alcaeus StudyStudy map
Overview / Summary
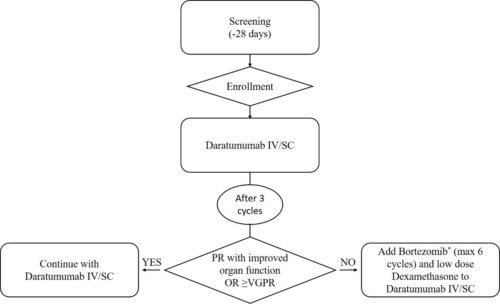
Study details
Patient eligibility criteria
| Men or women 18 years of age or older. |
| Diagnosis of amyloidosis, AL type, based on:
a. Histopathological diagnosis of amyloidosis based on detection by immunohistochemistry and polarizing light microscopy of green bi-refringent material in Congo Red stained tissue specimens (excluding bone marrow) or characteristic electron microscopy appearance Considerations for specific populations where other types of amyloidosis may be encountered: – For male subjects over 70 years of age who have cardiac involvement only, and subjects of African descent (black subjects), mass spectrometry, immunoelectron microscopy, or other immunohistochemistry-based typing of AL amyloid in a tissue biopsy or a negative bone scintigraphy with Tc99-PYP or –DPD is recommended to rule out other types of amyloidosis such as age related amyloidosis and/or hereditary amyloidosis (ATTR mutation) AND b. Measurable disease of amyloid light chain amyloidosis as defined by at least ONE of the following: – serum monoclonal protein ≥0.5 g/dL by protein electrophoresis (routine serum protein electrophoresis and immunofixation performed at local lab) – serum free light chain (FLC) ≥2.0 mg/dL (20 mg/L) with an abnormal kappa:lambda ratio or the difference between involved and uninvolved free light chains (dFLC) ≥2mg/dL (20 mg/L). Serum FLCs will be measured using the Freelite assay at a central laboratory. AND c. Cardiac involvement by AL amyloidosis according to consensus guidelines |
| Mayo Stage 3B disease, defined as both A. increased cardiac troponin (hsTnT >54 pg/ml) AND B. increased NT-proBNP ≥ 8500 pg/ml |
| For subjects with congestive heart failure, symptoms should be optimally managed and clinically stable with no cardiovascular-related hospitalizations within 2 weeks prior to Cycle 1 Day 1, as assessed by the Principal Investigator. |
| Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0, 1,2 or 3 |
| Subject must have pre-treatment clinical laboratory values meeting the following criteria during the Screening Phase:
a. Absolute neutrophil count ≥1.0 × 109/L; b. Hemoglobin level ≥10.0 g/dL (≥5 mmol/L) c. Platelet count ≥75 × 109/L; platelet transfusions are NOT acceptable d. Alanine aminotransferase level (ALT) ≤2.5 x ULN; e. Aspartate aminotransferase (AST) ≤2.5 x ULN f. Total bilirubin level ≤1.5 × ULN, except for subjects with history of Gilbert Syndrome, in which case direct bilirubin ≤ 2 × ULN g. Estimated Glomerular Filtration Rate (eGFR) ≥20 mL/min; Please note that the eGFR is measured by using the CKD-EPI equation |
| Women of childbearing potential must commit to either abstain continuously from heterosexual sexual intercourse (if this is the preferred and usual lifestyle of the subject) or to use 2 methods of reliable birth control simultaneously. This includes one highly effective form of contraception (tubal ligation, intrauterine device, hormonal [birth control pills, injections, hormonal patches, vaginal rings or implants] or partner’s vasectomy) and one additional effective contraceptive method (male latex or synthetic condom, diaphragm, or cervical cap). Contraception must begin prior to dosing and continue for 3 months after discontinuation of daratumumab. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy or bilateral oophorectomy. |
| During the study and for 3 months after receiving the last dose of daratumumab, female subjects must agree not to donate eggs (ova, oocytes) for the purposes of assisted reproduction. |
| A man who is sexually active with a woman of childbearing potential and has not had a vasectomy must agree to use a barrier method of birth control, e.g. either condom with spermicidal foam/gel/film/cream/suppository or partner with occlusive cap (diaphragm or cervical/vault caps) with spermicidal foam/gel/film/cream/suppository during and up to 3 months after discontinuation of daratumumab. All men must not donate sperm during the study and for 3 months after discontinuation of daratumumab. |
| Female subjects of childbearing potential must have a negative serum or urine pregnancy tests within 14 days prior to Cycle 1 Day 1. For requirements during the Treatment Phase, please see the Time and Events Schedule. |
| Each subject must sign an informed consent form (ICF) indicating that he or she understands the purpose of the procedures required for the study and are willing to participate in the study. Subjects must be willing and able to adhere to the prohibitions and restrictions specified in this protocol, as referenced in the ICF. |